Gut bacteria fuel tumor immune escape in esophageal cancer
GA, UNITED STATES, July 19, 2025 /EINPresswire.com/ -- A growing body of research is revealing the hidden influence of microbes on cancer. Recently, researchers have identified Fusobacterium nucleatum as a key player in shaping the immune environment in esophageal squamous cell carcinoma (ESCC). By integrating data from microbiome, metabolome, and transcriptome analyses, the study shows that the bacterium and an associated metabolite phenyllactic acid (PLA) promote the expression of CLEC12A, an immune receptor that encourages immune-suppressive macrophages. This dual microbial and molecular mechanism may contribute to treatment resistance in some ESCC patients, offering a potential explanation for the uneven response to immunotherapy and a new target for future interventions.
Esophageal squamous cell carcinoma (ESCC) is a deadly cancer with particularly high prevalence in East Asia. While immunotherapy has emerged as a promising treatment, many patients fail to respond—raising questions about hidden factors influencing therapeutic outcomes. Recent studies have turned attention to the tumor microbiome, which can interact with host immunity in complex ways. Oral pathogens such as F. nucleatum, long associated with periodontal disease, are now being implicated in cancer progression and immune escape. Their metabolites may serve as messengers, subtly rewiring the tumor's immune landscape. Due to these challenges, in-depth research is needed to uncover microbial mechanisms that modulate immune responses in ESCC.
In a letter-style study published on October 29, 2024, in Protein & Cell, a research team from the Fourth Hospital of Hebei Medical University, etc. revealed how Fusobacterium nucleatum manipulates immune behavior in ESCC. Drawing from a cohort of 52 patient samples, the team conducted microbiome sequencing, transcriptome analysis, and metabolomics profiling. Their work uncovered a strong link between microbial colonization, immune receptor expression, and immune cell polarization—offering fresh insight into how bacteria might undercut the body's cancer-fighting abilities.
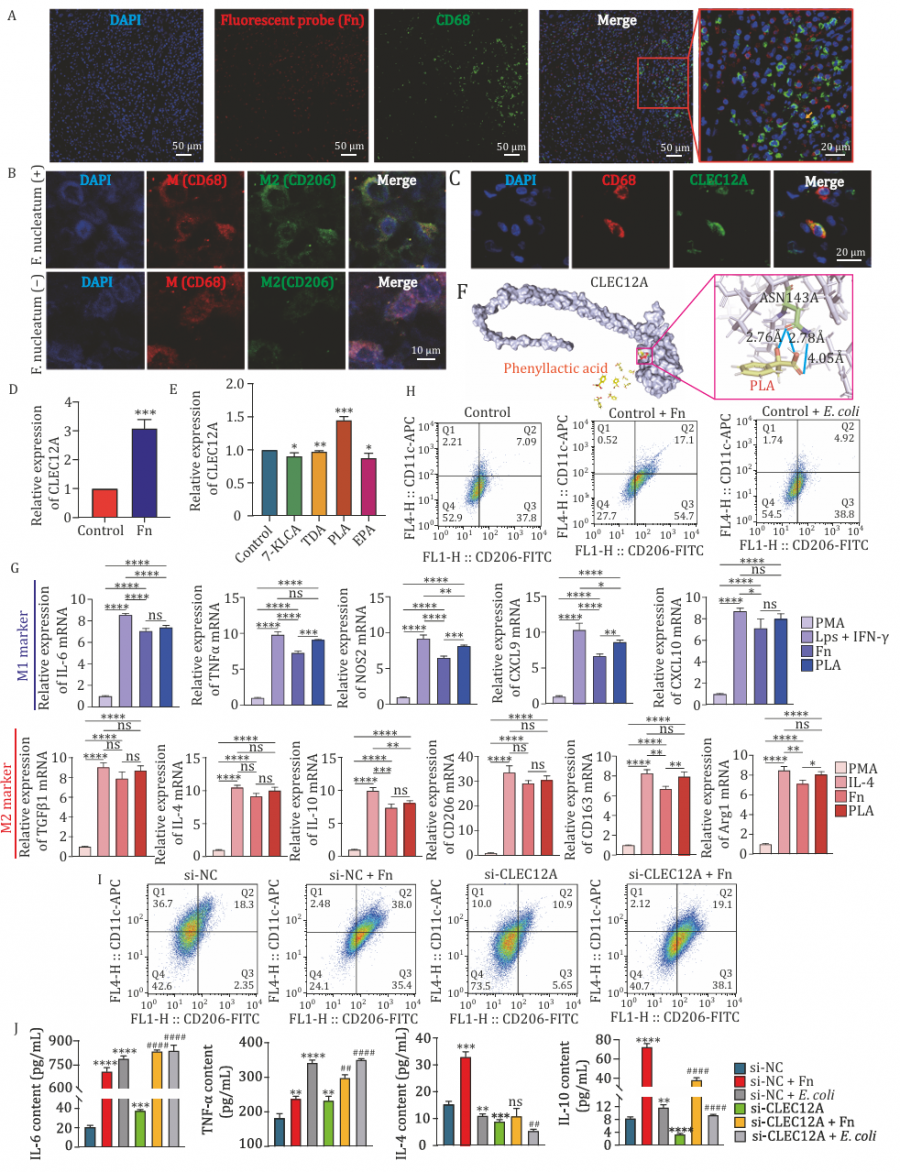
The researchers divided ESCC patients into treated and untreated groups and profiled their tumors at multiple molecular levels. They found that F. nucleatum was significantly more abundant in untreated tumors and was associated with more advanced cancer stages. Transcriptome analysis revealed that patients with high levels of this bacterium also had increased expression of CLEC12A, a receptor found on immune cells like macrophages. These immune cells, when influenced by F. nucleatum, shifted toward the M2 state—a pro-tumor phenotype characterized by heightened expression of immunosuppressive markers (e.g., CD206, CD163).
Further analysis pointed to phenyllactic acid (PLA), a metabolite associated with the bacterium, as a key driver of this effect. Lab experiments confirmed that both F. nucleatum and PLA upregulated CLEC12A and boosted M2 macrophage markers in cultured immune cells. Silencing CLEC12A reduced the bacterium's effect on M2 polarization and restored pro-inflammatory responses. These findings suggest a microbiota-driven mechanism that helps tumors escape immune surveillance by recruiting "tolerant" immune cells, and they highlight CLEC12A as a potential molecular bridge between microbial signals and immune modulation.
This study shines a light on the microbial dark matter of cancer, said Dr. Jing Zuo, one of the co-corresponding authors of the study. The research team has uncovered how F. nucleatum not only survives in tumor environments but actively changes how immune cells behave. By producing or associating with specific metabolites like PLA, the bacterium effectively reprograms macrophages into a tumor-supportive state. This opens up exciting possibilities for developing precision immunotherapy that takes a patient's microbiome into account.
The findings open new frontiers in the battle against ESCC. With F. nucleatum and PLA shown to influence immune evasion, the study points to microbiome profiling as a potential predictive tool for immunotherapy success. Therapies that disrupt this microbial-immunological axis—such as targeting CLEC12A or modifying gut flora—could offer innovative ways to overcome treatment resistance. Additionally, these insights could extend beyond ESCC, prompting researchers to investigate similar microbial effects in other cancers. As the interplay between microbes and immunity becomes clearer, the future of cancer therapy may hinge on more than just genetics—it may depend on our microbial cohabitants as well.
References
DOI
10.1093/procel/pwae063
Original Source URL
https://doi.org/10.1093/procel/pwae063
Funding information
This work was supported by the Natural Science Foundation of China (Grant No. 82373072) awarded to Z.N., the Key Project of Hebei Province Natural Science Precision Medicine Joint Fund (H2022201067) awarded to Z.N., and the Natural Science Foundation of Hebei Province (Grant No. H2024206159) awarded to J.Z.
Lucy Wang
BioDesign Research
email us here
Distribution channels: Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release