
Bacterial Vaginosis Market to Register Incremental Growth During the Study Period (2020–2034) | DelveInsight
The current therapeutic landscape of bacterial vaginosis is driven by approved therapies such as XACIATO, SOLOSEC, and VivaGel. The launch of novel developmental candidates currently in the emerging portfolio is expected to create a positive impact on the overall therapeutic landscape catering treatment options during the forecast period (2024–2034).
/EIN News/ -- New York, USA, July 15, 2024 (GLOBE NEWSWIRE) -- Bacterial Vaginosis Market to Register Incremental Growth During the Study Period (2020–2034) | DelveInsight
The current therapeutic landscape of bacterial vaginosis is driven by approved therapies such as XACIATO, SOLOSEC, and VivaGel. The launch of novel developmental candidates currently in the emerging portfolio is expected to create a positive impact on the overall therapeutic landscape catering treatment options during the forecast period (2024–2034).
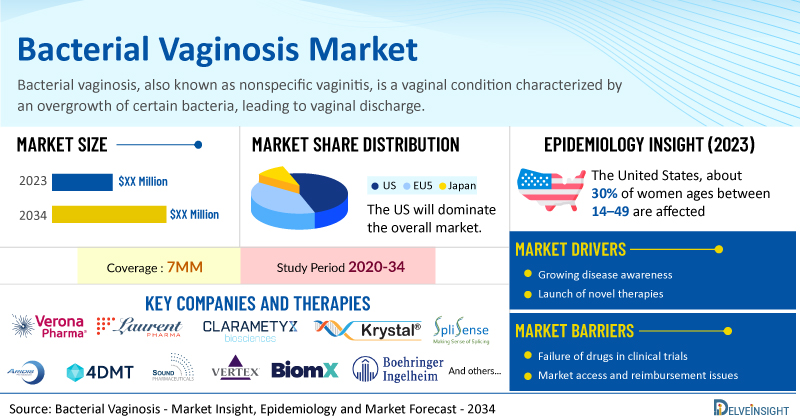
DelveInsight’s Bacterial Vaginosis Market Insights report includes a comprehensive understanding of current treatment practices, bacterial vaginosis emerging drugs, market share of individual therapies, and current and forecasted bacterial vaginosis market size from 2020 to 2034, segmented into 7MM [the United States, the EU-4 (Italy, Spain, France, and Germany), the United Kingdom, and Japan].
Key Takeaways from the Bacterial Vaginosis Market Report
- According to DelveInsight’s analysis, the market size of bacterial vaginosis in the 7MM is expected to grow at a significant CAGR by 2034.
- Bacterial vaginosis is the most common vaginal infection found in women of reproductive age and is estimated to occur in anywhere from 5–70% of women. In the United States, about 30% of women ages between 14–49 are affected, however, rates are variable between different ethnic groups and are most common in non-white women.
- Prominent companies working in the domain of bacterial vaginosis, including Osel, Siolta Therapeutics, Gedea Biotech, and others, are actively working on innovative drugs for bacterial vaginosis. These novel bacterial vaginosis therapies are anticipated to enter the bacterial vaginosis market in the forecast period and are expected to change the market.
- Some of the key therapies for bacterial vaginosis treatment include LACTIN-V, STMC-105, pHyph, and others.
Discover which therapies are expected to grab the bacterial vaginosis market share @ Bacterial Vaginosis Market Report
Bacterial Vaginosis Overview
Bacterial vaginosis, also known as nonspecific vaginitis, is a vaginal condition characterized by an overgrowth of certain bacteria, leading to vaginal discharge. It is the most common form of infectious vaginitis. The risk factors for bacterial vaginosis mirror those for sexually transmitted infections. Notable risk factors include antibiotic use, intrauterine devices for birth control, and cigarette smoking, all of which increase the likelihood of developing bacterial vaginosis.
Bacterial vaginosis can elevate the risk of several complications, such as pelvic inflammatory disease, endometritis post-abortion, postpartum, and posthysterectomy vaginal cuff infection. During pregnancy, it is linked to a higher risk of chorioamnionitis, premature rupture of membranes, preterm labor, and preterm birth.
Many women with bacterial vaginosis exhibit no symptoms. However, when symptoms are present, they typically include an abnormal amount of vaginal discharge, thin and grayish-white discharge, and a vaginal odor. These symptoms can occur at any point in the menstrual cycle, including before, during, or after menstruation.
Bacterial Vaginosis Epidemiology Segmentation
The bacterial vaginosis epidemiology section provides insights into the historical and current bacterial vaginosis patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The bacterial vaginosis market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
- Prevalent Cases of Bacterial Vaginosis
- Diagnosed Prevalent Cases of Bacterial Vaginosis
- Age-Specific Diagnosed Prevalent Cases of Bacterial Vaginosis
- Treatable Cases of Bacterial Vaginosis
Download the report to understand which factors are driving bacterial vaginosis epidemiology trends @ Bacterial Vaginosis Epidemiological Insights
Bacterial Vaginosis Treatment Market
The primary treatment options for bacterial vaginosis currently include metronidazole, clindamycin in either oral or vaginal suppository form, and metronidazole vaginal gel. Although up to 30% of BV cases may resolve on their own without treatment, the condition can be effectively treated with either clindamycin or metronidazole, both of which can be administered orally or vaginally. Additionally, these medications are safe for use during pregnancy. Secnidazole, which has been around since the late 1960s, is not widely known among US healthcare providers. The most recent addition to the US market is Solosec (secnidazole), which was approved in 2017 specifically for bacterial vaginosis. Solosec was the first therapy in the US to receive FDA approval for bacterial vaginosis and was designated as a Qualified Infectious Disease Product (QIDP) with Fast Track status.
In December 2021, Daré announced that the FDA approved XACIATO (clindamycin phosphate) vaginal gel, 2% (previously known as DARE-BV1) for treating bacterial vaginosis in females aged 12 and older. In June 2022, Organon, a global women’s healthcare company, and Daré Bioscience announced the completion of an agreement in which Organon acquired the global licensing rights to XACIATO (clindamycin phosphate) vaginal gel 2%.
Another authorized treatment is VivaGel BV, a mucoadhesive gel approved in Europe in 2019. It is the first non-antibiotic treatment for preventing recurrent BV. Consequently, it holds QIDP and Fast Track status for these indications in various markets.
Learn more about the FDA-approved drugs for bacterial vaginosis @ Drugs for Bacterial Vaginosis Treatment
Bacterial Vaginosis Emerging Drugs and Companies
The dynamics of the bacterial vaginosis treatment market are anticipated to change during the forecast period as companies across the 7MM are diligently working towards the development of novel treatment options to combat the existing treatment-based unmet needs. Key players, such as Osel, and other companies are involved in developing potential agents for the treatment of bacterial vaginosis.
Osel’s LACTIN-V is a live biotherapeutic product designed to address women's health issues. This product is being developed to prevent recurrent bacterial vaginosis and preterm birth. It contains Lactobacillus crispatus CTV-05, a strain of hydrogen peroxide-producing vaginal Lactobacillus that naturally occurs in the vaginal microbiome of many healthy women. LACTIN-V helps reduce recurrent bacterial vaginosis and preterm birth in high-risk women by promoting the colonization of L. crispatus. It is administered vaginally using a proprietary applicator. In 2019, LACTIN-V completed its Phase II clinical trial.
Siolta Therapeutics is currently in the preclinical phase with its candidate STMC-105, aiming to treat bacterial vaginosis. Meanwhile, Gedea Biotech received approval in November 2023 to commence the pivotal NEFERTITI-2 clinical trial, focusing on evaluating the effectiveness of pHyph in treating bacterial vaginosis.
The anticipated launch of these emerging therapies are poised to transform the bacterial vaginosis market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the bacterial vaginosis market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about bacterial vaginosis clinical trials, visit @ Bacterial Vaginosis Treatment Drugs
Bacterial Vaginosis Market Dynamics
The bacterial vaginosis market dynamics are anticipated to change in the coming years. The two factors projected to play a major role in shaping the bacterial vaginosis drug market are advanced scientific technology and sustainability, and various organizations such as the Women's Health Organization and the European Institute of Women’s Health (EIWH) have undertaken initiatives to increase awareness regarding women’s health.
Furthermore, many potential therapies are being investigated for the treatment of bacterial vaginosis, and it is safe to predict that the treatment space will significantly impact the bacterial vaginosis market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate are expected to drive the growth of the bacterial vaginosis market in the 7MM.
However, several factors may impede the growth of the bacterial vaginosis market. Greater awareness of bacterial vaginosis is needed across various groups, including women and healthcare providers (HCPs) because it is a condition that can have serious health implications such as pre-term labor, pelvic inflammatory disease, and increased transmission of HIV and other sexually transmitted infections.
Moreover, bacterial vaginosis treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the bacterial vaginosis market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the bacterial vaginosis market growth.
| Bacterial Vaginosis Report Metrics | Details |
| Study Period | 2020–2034 |
| Bacterial Vaginosis Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Key Bacterial Vaginosis Companies | Osel, Siolta Therapeutics, Gedea Biotech, and others |
| Key Bacterial Vaginosis Therapies | LACTIN-V, STMC-105, pHyph, and others |
Scope of the Bacterial Vaginosis Market Report
- Bacterial Vaginosis Therapeutic Assessment: Bacterial Vaginosis current marketed and emerging therapies
- Bacterial Vaginosis Market Dynamics: Attribute Analysis of Emerging Bacterial Vaginosis Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Bacterial Vaginosis Market Access and Reimbursement
Discover more about bacterial vaginosis drugs in development @ Bacterial Vaginosis Clinical Trials
Table of Contents
| 1. | Bacterial Vaginosis Market Key Insights |
| 2. | Bacterial Vaginosis Market Report Introduction |
| 3. | Bacterial Vaginosis Market Overview at a Glance |
| 4. | Bacterial Vaginosis Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Bacterial Vaginosis Treatment and Management |
| 7. | Bacterial Vaginosis Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Bacterial Vaginosis Marketed Drugs |
| 10. | Bacterial Vaginosis Emerging Drugs |
| 11. | Seven Major Bacterial Vaginosis Market Analysis |
| 12. | Bacterial Vaginosis Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
| 17. | Appendix |
| 18. | DelveInsight Capabilities |
| 19. | Disclaimer |
| 20. | About DelveInsight |
Related Reports
Bacterial Vaginosis Epidemiology Forecast
Bacterial Vaginosis Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted bacterial vaginosis epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Bacterial Vaginosis Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key bacterial vaginosis companies, including Osel Inc, Evofem Biosciences, LUCA Biologics, Melinta Therapeutics, TenNor Therapeutics, Mayfield Pharmaceuticals, Toltec Pharmaceuticals, among others.
HIV Type-1 Market Insight, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key HIV type-1 companies, including MacroGenics, CytoDyn, Inc., ViiV Healthcare, GlaxoSmithKline, PPD, Excision BioTherapeutics, TaiMed Biologics Inc., Gilead Sciences, ST Pharm Co., Ltd., Merck Sharp & Dohme LLC, Janssen Sciences Ireland UC, Vir Biotechnology, Inc., National Institute of Allergy and Infectious Diseases (NIAID), AbbVie, Sanofi, ModeX Therapeutics, among others.
HIV Type-1 Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key HIV type-1 companies, including United BioPharma, Gilead Sciences, Taimed Biologics, Merck Sharp &Dohme, Janssen Sciences, CytoDyn Biosciences, MacroGenics, among others.
HIV-associated Lipodystrophy Market
HIV-associated Lipodystrophy Market Insights, Epidemiology, and Market Forecast – 2032 report delivers an in-depth understanding of the market trends, market drivers, market barriers, and key HIV-associated lipodystrophy companies, including Gilead Sciences, Amgen, Abbott, AstraZeneca, AbbVie, GlaxoSmithKline, Alfa Wassermann SPA, Theratechnologies, Bristol-Myers Squibb, among others.
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
EIN Presswire does not exercise editorial control over third-party content provided, uploaded, published, or distributed by users of EIN Presswire. We are a distributor, not a publisher, of 3rd party content. Such content may contain the views, opinions, statements, offers, and other material of the respective users, suppliers, participants, or authors.


