Eupraxia Pharmaceuticals Announces Sustained Positive Treatment Outcomes in Patients with Eosinophilic Esophagitis (EoE) After Nine Months of Receiving EP-104GI
- Eupraxia continues to observe positive treatment outcomes in its ongoing Phase 1b/2a trial, including for the first time clinical responses measured 9 months after dosing with EP-104GI
- At 9 months active drug continued to be released into the esophagus in patients at a rate similar to what was seen at the 3- and 6-month time points. This is an unprecedented result with an injectable delivery system in patients with EoE
- Also, at 9 months patients demonstrated sustained or improved treatment outcomes compared to 3- and 6-month results
- Additional long-term data to be released with higher doses in Q3 2025
- Eupraxia to host webinar with guest, Dr. Evan Dellon, to discuss data from RESOLVE trial on Friday, May 9th at 9:00am ET (Register here)
/EIN News/ -- VICTORIA, British Columbia, May 05, 2025 (GLOBE NEWSWIRE) -- Eupraxia Pharmaceuticals Inc. (“Eupraxia” or the “Company”) (NASDAQ:EPRX) (TSX:EPRX), a clinical-stage biotechnology company leveraging its proprietary DiffuSphere™ technology designed to optimize drug delivery for applications with significant unmet need, today announced positive treatment outcomes from its ongoing RESOLVE Phase 1b/2a trial evaluating EP-104GI for the treatment of eosinophilic esophagitis ("EoE"). Most notably, nine-month data was disclosed for the first time demonstrating that all three patients dosed at 48mg of EP-104GI experienced sustained or improved treatment outcomes after nine months of therapy.
“EoE is a chronic medical condition and novel treatment options are needed to effectively manage the long-term tissue inflammation and other associated symptoms of EoE,” said Dr. Evan S. Dellon, MD, MPH (University of North Carolina School of Medicine) and Chairman of the Company's Gastrointestinal Clinical Advisory Board. “The durability in symptom reduction coupled with the prolonged improvement in tissue health from the ongoing RESOLVE trial suggest that EP-104GI could be exerting a tissue remodeling effect on the esophagus. Based on these encouraging results, I look forward to EP-104GI continuing to advance in the clinic, as patients with EoE are in need of new treatment options with disease-modifying potential.”
“Based on the unprecedented durability in symptom improvements we are now observing in the RESOLVE study, we believe EP-104GI has the potential to significantly improve upon the current standard of care for EoE,” said Dr. James Helliwell, Chief Executive Officer of Eupraxia. “After receiving treatment with EP-104GI nine months earlier, all patients in the trial dosed at 48mg continue to experience sustained or improved treatment outcomes across multiple clinical measurements, including symptom improvement, tissue health, and reductions in eosinophil count. It is also important to note that patients received 48mg EP-104GI which is 50% of the potential dose per site and covers only the lower two-thirds of the esophagus. As we further dose optimize EP-104GI and expand therapeutic coverage of the esophagus, we have the opportunity to potentially realize even further improvements on top of these already remarkable and promising results.”
EoE is an inflammatory-mediated disease in which white blood cells migrate into and become trapped in the esophagus, creating pain and difficulty with swallowing food. The Company’s ongoing RESOLVE trial is a Phase 1b/2a, multi-center, open-label, dose-escalation study that is evaluating EP-104GI across multiple patient cohorts by assessing key clinical measurements associated with EoE, including Straumann Dysphagia Index (“SDI”), impact on tissue health (histology) as measured by EoEHSS (“Eosinophilic Esophagitis Histology Scoring System”), and the measurement of peak eosinophil count ("PEC").
Key Findings from the 9-month data for Cohort 5 of the RESOLVE trial
Nine-month data was collected for the first time in the RESOLVE study in Cohort 5. Each patient in Cohort 5 received 12 injections of 4 mg EP-104GI (total dose: 48 mg) delivered to the lower two-thirds of the esophagus. Results at 36 weeks include:
- Symptom Improvement (SDI): All three patients reported reduced symptom severity. Peak improvements reached up to a 5-point reduction (100%), with an average reduction of 3.7 points (65%) on the SDI.
- Tissue Health (EoEHSS): Improvements in esophageal tissue health were sustained, with an average 56% reduction in EoEHSS Stage score (-0.34) and a 45% reduction in Grade score (-0.25).
- Reductions in Eosinophils: Reductions in eosinophil counts were maintained, with a mean decrease of 77% across a standard number of biopsy sites within the treated area. The mean remission rate across all biopsy sites was 52%. Notably, one patient maintained complete histologic remission—defined as all sites showing less than or equal to 6 eosinophils per high-power field—originally achieved at 3 months and sustained through 9 months.
- Safety outcomes: Plasma levels of fluticasone remained steady and predictable, well below levels typically observed with daily asthma inhalers. No serious adverse events were reported.
Cohort 5 data at 12 & 36 weeks
| Measurement | 12 weeks | 36 weeks | |
| SDI mean improvement | 2.3 | 3.7 | |
|
PEC |
Response Rate (<15 eos per HPF) | 48% | 60% |
| Remission Rate (< 6 eos per HPF) |
38% | 52% | |
|
EoEHSS |
Extent (stage) | -0.33 (54%) | -0.34 (56%) |
| Magnitude (grade) | -0.31 (54%) | -0.25 (45%) | |
EP-104GI Pharmacokinetics - week 36 levels remain steady and comparable to week 12
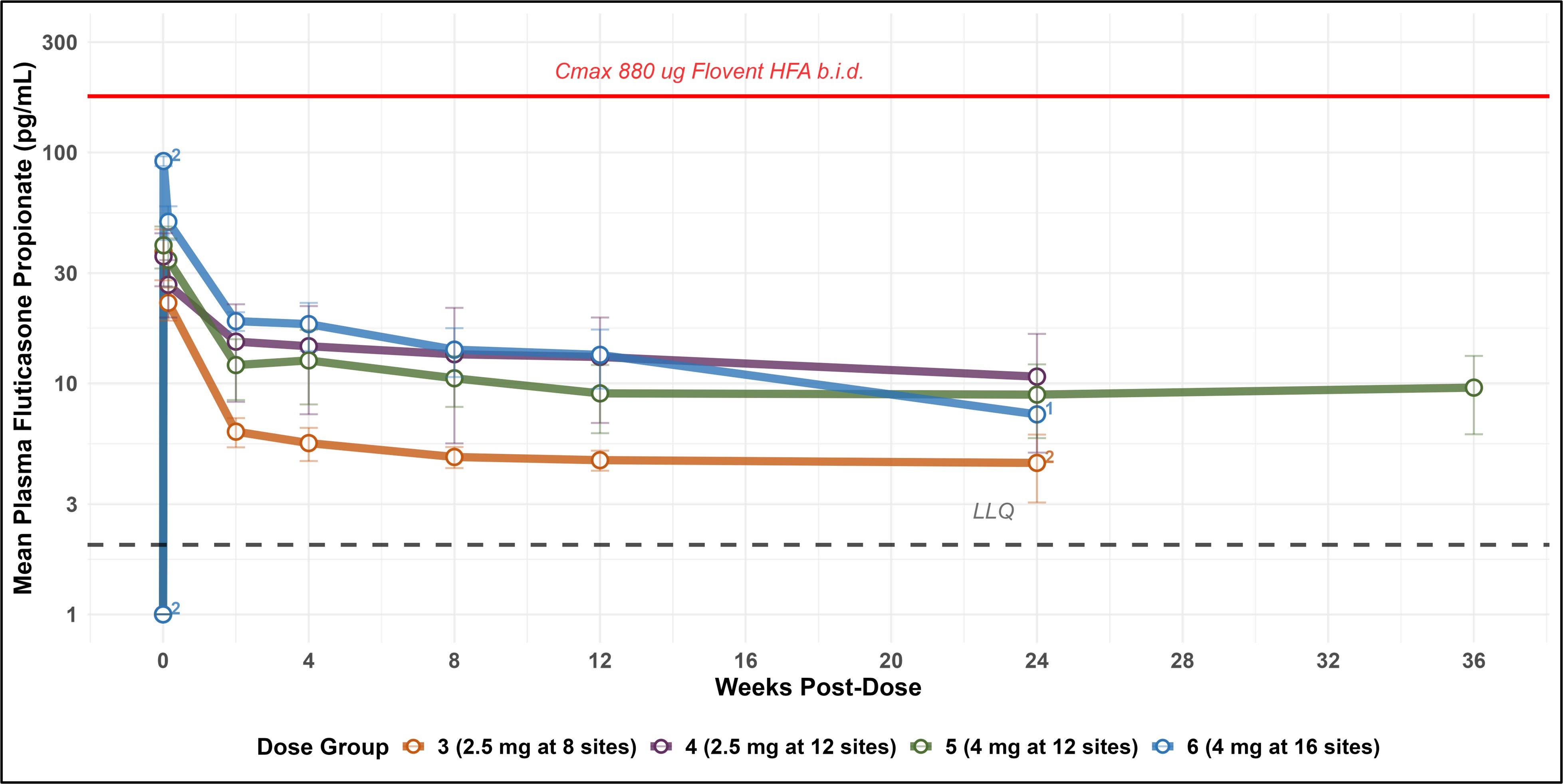
Key Findings from the 6-month data for Cohort 6 of the RESOLVE Trial
Each patient in the sixth cohort received 16 injections of 4 mg EP-104GI (total dose: 64 mg) targeting the lower three-quarters of the esophagus. The results include:
-
Symptom Improvement (SDI): All three patients reported reduced symptom severity, with peak SDI score reductions of up to 6 points (86%) and an average reduction of 4.3 points (67%) at 24 weeks.
** EoEHSS is a composite score of various histological inflammatory measures, split into separate metrics of "stage" (extent) and "grade" (severity).
Webinar to discuss results
The Company will host a webinar with guest, Dr. Evan Dellon, to discuss data from the RESOLVE trial on Friday, May 9th at 9:00am ET. Please click on the link below to register for the webinar:
Register here.
About the RESOLVE Trial
The RESOLVE trial is a Phase 1b/2a, multicenter, open-label, dose-escalation study evaluating the safety, tolerability, pharmacokinetics, and efficacy of EP-104GI in adults with histologically confirmed active EoE. The treatment is administered as a single dose via four to 20 esophageal wall injections, with dose escalations modifying either the dose per site or the number of sites. Patients in Cohorts 1–4 were evaluated for up to 24 weeks, while patients in Cohorts 5 and beyond are assessed for up to 52 weeks. Eupraxia plans to disclose additional data periodically.
Additional long-term data to be released with higher doses in Q3 2025.
Notes
- SDI is a patient-reported outcome score that uses a seven-day recall measuring dysphagia (trouble swallowing) severity and frequency. A reduction in SDI is a positive outcome for the RESOLVE trial.
- In the EoEHSS, grade indicates the severity of each of the eight histologic features assessed by the EoEHSS while stage indicates their extent. For the RESOLVE trial, these features include inflammation, increased cell production in a normal tissue or organ, and fibrosis, also known as fibrotic scarring, and five other features. A reduction in EoEHSS is a positive outcome for the RESOLVE trial.
- PEC means the peak number of eosinophils found in esophageal biopsies. Eosinophils are one of several white blood cells that support a person’s immune system. A reduction in PEC is a positive outcome for the RESOLVE trial. If a biopsy site has less than or equal to 6 eosinophils, that site is considered to be in remission. Remission Rate is the percentage of biopsies that are in remission.
About EoE
EoE is an inflammatory-mediated disease in which white blood cells migrate into and become trapped in the esophagus, creating pain and difficulty with swallowing food. According to market research from Clearview Healthcare Partners, EoE affects more than 450,000 people in the United States and has been identified by the American Gastroenterological Association as rapidly increasing in both incidence and prevalence. Impacts from both symptoms and interventions frequently lead to mental health issues, compounding the disease burden of EoE for both the healthcare system and the individual.
About Eupraxia Pharmaceuticals Inc.
Eupraxia is a clinical-stage biotechnology company focused on the development of locally delivered, extended-release products that have the potential to address therapeutic areas with high unmet medical need. DiffuSphere™, a proprietary, polymer-based micro-sphere technology, is designed to facilitate targeted drug delivery of both existing and novel drugs. The technology is designed to support extended duration of effect and delivery of drugs in a hyper-localized fashion, targeting only the tissues that physicians are wanting to treat. We believe the potential for fewer adverse events may be achieved through the precision targeting and the stable and flat delivery of the active ingredient when using the DiffuSphere™ technology, versus the peaks and troughs seen with more traditional drug delivery methods. The precision of Eupraxia's DiffuSphere™ technology platform has the potential to augment and transform existing FDA-approved drugs to improve their safety, tolerability, efficacy and duration of effect. The potential uses in therapeutic areas may go beyond pain and inflammatory gastrointestinal disease, where Eupraxia currently is developing advanced treatments, to also be applicable in oncology, infectious disease and other critical disease areas.
Eupraxia's EP-104GI is currently in a Phase 1b/2a trial, the RESOLVE trial, for the treatment of EoE. EP-104GI is administered as an injection into the esophageal wall, providing local delivery of drug. This is a unique treatment approach for EoE. Eupraxia also recently completed a Phase 2b clinical trial (SPRINGBOARD) of EP-104IAR for the treatment of pain due to knee osteoarthritis. The trial met its primary endpoint and three of the four secondary endpoints. In addition, Eupraxia is developing a pipeline of later and earlier-stage long-acting formulations. Potential pipeline indications include candidates for other inflammatory joint indications and oncology, each designed to improve on the activity and tolerability of currently approved drugs. For further details about Eupraxia, please visit the Company's website at: www.eupraxiapharma.com.
Notice Regarding Forward-looking Statements and Information
This news release includes forward-looking statements and forward-looking information within the meaning of applicable securities laws. Often, but not always, forward-looking information can be identified by the use of words such as "plans", "is expected", "expects", "suggests", "scheduled", "intends", "contemplates", "anticipates", "believes", "proposes", "potential" or variations (including negative and grammatical variations) of such words and phrases, or state that certain actions, events or results "may", "could", "would", "might" or "will" be taken, occur or be achieved. Forward-looking statements in this news release include statements regarding the release of additional long-term data with higher doses and timing thereof; the Company's product candidates, including their expected benefits to patients with respect to safety, tolerability, efficacy and duration; the results gathered from studies and trials of Eupraxia's product candidates; the potential for the Company’s technology to impact the drug delivery process; potential market opportunity for the Company’s products; and potential pipeline indications. Such statements and information are based on the current expectations of Eupraxia's management, and are based on assumptions, including but not limited to: future research and development plans for the Company proceeding substantially as currently envisioned; industry growth trends, including with respect to projected and actual industry sales; the Company's ability to obtain positive results from the Company's research and development activities, including clinical trials; and the Company's ability to protect patents and proprietary rights. Although Eupraxia's management believes that the assumptions underlying these statements and information are reasonable, they may prove to be incorrect. The forward-looking events and circumstances discussed in this news release may not occur by certain dates or at all and could differ materially as a result of known and unknown risk factors and uncertainties affecting Eupraxia, including, but not limited to: risks and uncertainties related to the Company's limited operating history; the Company's novel technology with uncertain market acceptance; if the Company breaches any of the agreements under which it licenses rights to its product candidates or technology from third parties, the Company could lose license rights that are important to its business; the Company's current license agreement may not provide an adequate remedy for its breach by the licensor; the Company's technology may not be successful for its intended use; the Company's future technology will require regulatory approval, which is costly and the Company may not be able to obtain it; the Company may fail to obtain regulatory approvals or only obtain approvals for limited uses or indications; the Company's clinical trials may fail to demonstrate adequately the safety and efficacy of its product candidates at any stage of clinical development; the Company may be required to suspend or discontinue clinical trials due to side effects or other safety risks; the Company completely relies on third parties to provide supplies and inputs required for its products and services; the potential impact of tariffs on the cost of the Company’s API and clinical supplies of EP-104IAR and EP-104GI; the Company relies on external contract research organizations to provide clinical and non-clinical research services; the Company may not be able to successfully execute its business strategy; the Company will require additional financing, which may not be available; any therapeutics the Company develops will be subject to extensive, lengthy and uncertain regulatory requirements, which could adversely affect the Company's ability to obtain regulatory approval in a timely manner, or at all; the impact of health pandemics or epidemics on the Company's operations; the Company's restatement of its consolidated financial statements, which may lead to additional risks and uncertainties, including loss of investor confidence and negative impacts on the Company's common share price; and other risks and uncertainties described in more detail in Eupraxia's public filings on SEDAR+ (sedarplus.ca) and EDGAR (sec.gov). Although Eupraxia has attempted to identify important factors that could cause actual actions, events or results to differ materially from those described in forward-looking statements and information, there may be other factors that cause actions, events or results to differ from those anticipated, estimated or intended. No forward-looking statement or information can be guaranteed. Except as required by applicable securities laws, forward-looking statements and information speak only as of the date on which they are made and Eupraxia undertakes no obligation to publicly update or revise any forward-looking statement or information, whether as a result of new information, future events or otherwise.
For investor and media inquiries, please contact:
Danielle Egan, Eupraxia Pharmaceuticals Inc.
778.401.3302
degan@eupraxiapharma.com
or
Kevin Gardner, on behalf of:
Eupraxia Pharmaceuticals Inc.
617.283.2856
kgardner@lifesciadvisors.com
SOURCE Eupraxia Pharmaceuticals Inc.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/72fe2808-9a43-41cf-b914-49fd60aaace0

Distribution channels: Healthcare & Pharmaceuticals Industry, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release