
Autoinjectors Market is expected to generate a revenue of USD 317.01 Billion by 2031, Globally, at 23.20% CAGR: Verified Market Research®
Strategically, the autoinjectors market offers strong growth potential driven by rising chronic disease prevalence, biologics adoption, and patient demand for home-based care. However, high costs, technical risks, and regulatory hurdles require calculated entry. Companies targeting North America can leverage established reimbursement systems and patient awareness to scale quickly. Market entry strategies should focus on product reliability, cost-efficiency, and regulatory alignment. Strategic partnerships with pharma firms and investments in smart autoinjector innovation can unlock competitive advantages and long-term growth.
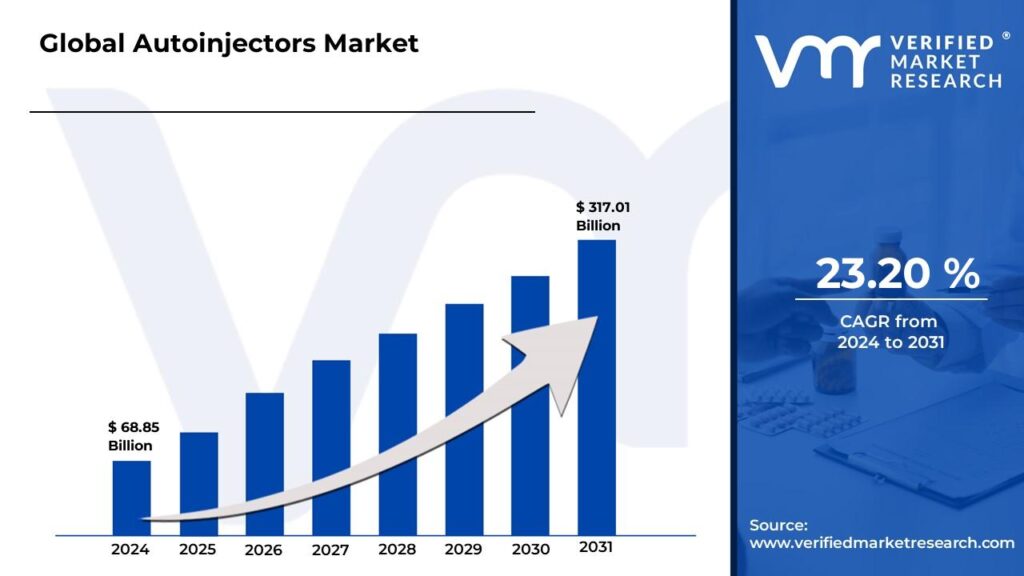
/EIN News/ -- Lewes, Delaware, May 20, 2025 (GLOBE NEWSWIRE) -- The Global Autoinjectors Market Size is projected to grow at a CAGR of 23.20% from 2024 to 2031, according to a new report published by Verified Market Research®. The report reveals that the market was valued at USD 68.85 Billion in 2024 and is expected to reach USD 317.01 Billion by the end of the forecast period.
The autoinjectors market is experiencing strong growth due to increasing patient preference for homecare, a rise in biologics usage, and growing chronic disease prevalence. Market players are innovating to overcome device complexity and cost barriers.
Key Highlights of the Report:
- Market Size & Forecast – In-depth analysis of current value and future projections.
- Segment Analysis – Detailed study across Type, Application, Route of Administration, and Distribution Channel.
- Regional Insights – Comprehensive coverage of North America, Europe, Asia-Pacific, and more.
- Competitive Landscape – Profiles of top players and their strategic initiatives.
- Technology Trends – Covers smart autoinjectors, Bluetooth-enabled devices, and design improvements.
- Regulatory Impact – Assessment of global and regional compliance frameworks.
Why This Report Matters:
This report offers comprehensive insights into current and emerging trends, enabling strategic planning for stakeholders. It delivers data-backed forecasts, competitive analysis, and regulatory updates, helping businesses mitigate risks and capture growth opportunities in the dynamic autoinjectors space.
Who You Should Read This Report:
- Medical Device Manufacturers – To identify innovation opportunities and product development trends.
- Pharmaceutical Companies – To align drug delivery innovations with market demands.
- Healthcare Providers – To understand patient-centric delivery systems.
- Investors & Consultants – For evaluating market potential and guiding investment decisions.
-
Regulatory Bodies – To assess safety, compliance, and innovation benchmarks in autoinjector devices.
 For more information or to purchase the report, please contact us at: https://www.verifiedmarketresearch.com/download-sample?rid=27669
For more information or to purchase the report, please contact us at: https://www.verifiedmarketresearch.com/download-sample?rid=27669
Browse in-depth TOC on “Global Autoinjectors Market Size”
202 - Pages
126 – Tables
37 – Figures
Report Scope
| REPORT ATTRIBUTES | DETAILS |
| STUDY PERIOD | 2021-2031 |
| BASE YEAR | 2024 |
| FORECAST PERIOD | 2024-2031 |
| HISTORICAL PERIOD | 2021-2023 |
| UNIT | Value (USD Billion) |
| KEY COMPANIES PROFILED | Ypsomed, Abbvie, Amgen, Teva, AstraZeneca, Biogen, Eli Lilly, Merck, Mylan, and SHL Group. |
| SEGMENTS COVERED | By Type, By Application, By Route of Administration, By Distribution Channel, And By Geography. |
| CUSTOMIZATION SCOPE | Free report customization (equivalent up to 4 analyst’s working days) with purchase. Addition or alteration to country, regional & segment scope |
Global Autoinjectors Market Overview
Market Driver
Rising Prevalence of Chronic and Autoimmune Diseases: The rising global prevalence of chronic and autoimmune diseases—such as diabetes, multiple sclerosis, and rheumatoid arthritis—constitutes a principal catalyst for the autoinjectors industry. These illnesses frequently necessitate prolonged, consistent administration of injectable pharmaceuticals. Autoinjectors provide secure, precise, and simple self-administration of biologics at home, diminishing reliance on clinical visits and enhancing treatment adherence. This corresponds with healthcare systems' emphasis on cost-efficient, patient-centered care delivery strategies, hence enhancing demand.
Increasing Demand for Self-Administration and Homecare Solutions: A notable transition in healthcare provision is occurring from hospital-centric treatment to homecare and self-administered therapies. Patients currently pursue medical solutions that provide confidentiality, ease, and adaptability. Autoinjectors address this requirement with prefilled, disposable, and user-friendly designs that obviate the necessity for expert administration. As knowledge of needle fear and infection concerns increases, autoinjectors are increasingly utilized in chronic care, emergency allergic reactions (e.g., epinephrine), and hormonal therapies, hence speeding market expansion.
Expansion of Biologic Drug Approvals and Monoclonal Antibodies: Biologic pharmaceuticals, particularly monoclonal antibodies (mAbs), are swiftly obtaining regulatory authorization in prominent healthcare markets. Due to the necessity for regular and accurate subcutaneous or intramuscular injection of these biologics, autoinjectors are emerging as the preferred delivery system. Pharmaceutical companies are using autoinjectors into medicine-device combo products to improve treatment efficacy and promote patient adherence. This tendency not only fosters innovation in therapeutic alternatives but also generates a sustained demand for sophisticated, user-friendly autoinjector systems.
 To Purchase a Comprehensive Report Analysis: https://www.verifiedmarketresearch.com/select-licence?rid=27669
To Purchase a Comprehensive Report Analysis: https://www.verifiedmarketresearch.com/select-licence?rid=27669
Market Restraint
High Cost of Autoinjectors and Associated Therapy: Notwithstanding their therapeutic advantages, autoinjectors continue to be costly, particularly those tailored for biologic medications. The whole expense encompasses device production, prefilled medication cartridges, and ongoing supply requirements. For patients lacking extensive insurance coverage or residing in low-income areas, cost becomes a substantial obstacle. Furthermore, healthcare providers frequently exhibit reluctance to use expensive delivery methods until they demonstrate quantifiable enhancements in patient outcomes. This cost sensitivity limits extensive market penetration, particularly in emerging economies.
Technical Failures, Product Recalls, and Safety Concerns: Autoinjectors, although engineered for user convenience, are susceptible to technical failures like incomplete dosage administration, needle obstruction, or device leakage. Such failures may jeopardize patient safety and the efficacy of treatment. Regulatory agencies such as the FDA and EMA have documented numerous product recalls due to quality concerns, prompting apprehensions regarding device reliability. B2B purchasers, particularly hospitals and payers, require rigorous quality audits and risk-mitigation methods, which impede purchasing choices and brand adoption.
Stringent Regulatory Requirements and Approval Delays: Autoinjectors are categorized as drug-device combination items, necessitating comprehensive testing for mechanical reliability, dose accuracy, and biocompatibility. The regulatory processes for these products are intricate and differ across regions, frequently resulting in prolonged approval periods. Moreover, continual alterations in compliance mandates, particularly with digital health integration in smart autoinjectors, contribute to increased development expenses and delays in time-to-market. These regulatory impediments pose a significant obstacle for both startups and established entities seeking swift commercialization.
Geographical Dominance
North America leads the autoinjectors market owing to its sophisticated healthcare infrastructure, significant uptake of biologics, and increasing incidence of chronic diseases. Robust reimbursement schemes, advantageous regulatory rules, and vigorous R&D investments by prominent pharmaceutical corporation’s further bolster regional growth. The United States excels in patient awareness and exhibits robust demand for self-administration technologies, establishing the region as a significant revenue source.
Key Players
The study report on the "Global Autoinjectors Market" will offer valuable insights, focusing specifically on the global market. The major players in the market are Ypsomed, Abbvie, Amgen, Teva, AstraZeneca, Biogen, Eli Lilly, Merck, Mylan, and SHL Group.
Autoinjectors Market Segment Analysis
Based on the research, Verified Market Research has segmented the global market into Type, Application, Route of Administration, Distribution Channel, and Geography.
-
Autoinjectors Market, by Type
- Disposable
- Reusable
-
Autoinjectors Market, by Application
- Autoimmune Disorders
- Diabetes
- Emergency Care
-
Autoinjectors Market, by Route of Administration
- Intramuscular
- Subcutaneous
-
Autoinjectors Market, by Distribution Channel
- Hospitals Pharmacies
- Retail Pharmacies
- Online Pharmacies
-
Autoinjectors Market, by Geography
-
North America
- U.S
- Canada
- Mexico
-
Europe
- Germany
- France
- U.K
- Rest of Europe
-
Asia Pacific
- China
- Japan
- India
- Rest of Asia Pacific
-
ROW
- Middle East & Africa
- Latin America
-
North America
Browse Related Reports:
Global Intraocular Lens Injectors Market Size By Type (Preloaded Intraocular Lens Injectors, Non-Preloaded Intraocular Lens Injectors), By IOL Compatibility (Hydrophilic, Hydrophobic), By Tip Incision Sizes (8mm, 0mm), By End User (Hospitals And Ambulatory Surgical Centres, Ophthalmic Clinics), By Geography, And Forecast
Global Wearable Injectors Market Size By Type (On-Body Injectors, Off-Body Injectors, Hand Held), By Therapy (Immuno-Oncology, Diabetes, Cardiovascular Diseases), By End-User (Hospitals, Home Care Settings, Clinics), By Geography, And Forecast
Global Vaccine Delivery Devices Market Size By Devices (Syringes, Jet Injectors), By Route Of Administration (Intradermal Vaccination, Intramuscular Vaccination, Subcutaneous Vaccination), By End-User (Hospitals And Clinics, Home Care Settings, Ambulatory Surgery Centers), By Geography, And Forecast
Global Contrast Media Injectors Market Size By Type (Single Head, Dual Head), By Product (Consumables, Injector Systems), By Application (Radiology, Interventional Radiology), By End-User (Hospitals, Diagnostic Imaging Centers), By Geography, And Forecast
Top 10 Synthetic Polymer Manufacturers transforming products with inventions
Visualize Autoinjectors Market using Verified Market Intelligence -:
Verified Market Intelligence is our BI Enabled Platform for narrative storytelling in this market. VMI offers in-depth forecasted trends and accurate Insights on over 20,000+ emerging & niche markets, helping you make critical revenue-impacting decisions for a brilliant future.
VMI provides a holistic overview and global competitive landscape with respect to Region, Country, Segment, and Key players of your market. Present your Market Report & findings with an inbuilt presentation feature saving over 70% of your time and resources for Investor, Sales & Marketing, R&D, and Product Development pitches. VMI enables data delivery In Excel and Interactive PDF formats with over 15+ Key Market Indicators for your market.
About Us
Verified Market Research® stands at the forefront as a global leader in Research and Consulting, offering unparalleled analytical research solutions that empower organizations with the insights needed for critical business decisions. Celebrating 10+ years of service, VMR has been instrumental in providing founders and companies with precise, up-to-date research data.
With a team of 500+ Analysts and subject matter experts, VMR leverages internationally recognized research methodologies for data collection and analyses, covering over 15,000 high impact and niche markets. This robust team ensures data integrity and offers insights that are both informative and actionable, tailored to the strategic needs of businesses across various industries.
VMR's domain expertise is recognized across 14 key industries, including Semiconductor & Electronics, Healthcare & Pharmaceuticals, Energy, Technology, Automobiles, Defense, Mining, Manufacturing, Retail, and Agriculture & Food. In-depth market analysis cover over 52 countries, with advanced data collection methods and sophisticated research techniques being utilized. This approach allows for actionable insights to be furnished by seasoned analysts, equipping clients with the essential knowledge necessary for critical revenue decisions across these varied and vital industries.
Verified Market Research® is also a member of ESOMAR, an organization renowned for setting the benchmark in ethical and professional standards in market research. This affiliation highlights VMR's dedication to conducting research with integrity and reliability, ensuring that the insights offered are not only valuable but also ethically sourced and respected worldwide.

Mr. Edwyne Fernandes
Verified Market Research®
US: +1 (650)-781-4080
US Toll Free: +1 (800)-782-1768
Email: sales@verifiedmarketresearch.com
Web: https://www.verifiedmarketresearch.com/
Follow Us: LinkedIn | Twitter | Threads | Instagram | Facebook
SOURCE – Verified Market Research®
Distribution channels: Business & Economy, Media, Advertising & PR ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

